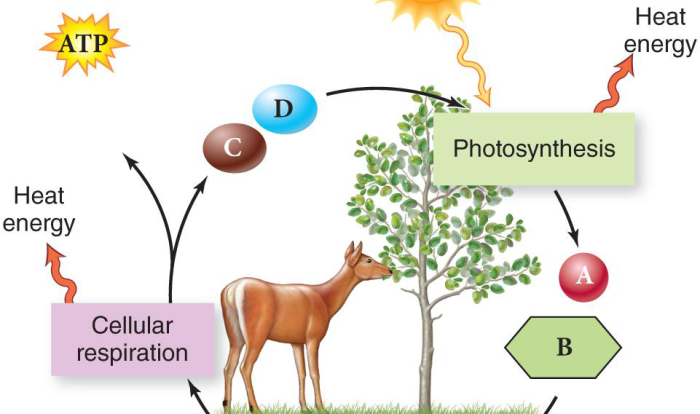
Introducing the osmosis and diffusion venn diagram, an innovative tool that unveils the intricate relationship between two fundamental biological processes. This diagram not only provides a clear visual representation but also serves as a gateway to understanding the mechanisms that govern the movement of molecules across membranes.
Throughout this discussion, we will delve into the similarities and differences between osmosis and diffusion, exploring their unique characteristics and shared features. Prepare to embark on a journey that will shed light on these essential processes and their profound impact on living organisms.
Osmosis and Diffusion Definitions
Osmosis and diffusion are two fundamental processes that govern the movement of molecules across biological membranes. These processes play crucial roles in various biological functions, including the exchange of nutrients, waste products, and water between cells and their surroundings.
Osmosis, Osmosis and diffusion venn diagram
Osmosis is the movement of water molecules across a semipermeable membrane from an area of high water concentration to an area of low water concentration. A semipermeable membrane is a barrier that allows water molecules to pass through but prevents the passage of other substances.
Osmosis occurs in response to a difference in water potential, which is a measure of the tendency of water to move from one area to another.
Diffusion
Diffusion is the movement of molecules from an area of high concentration to an area of low concentration. Unlike osmosis, diffusion does not require a semipermeable membrane and can occur across any medium. Molecules move down their concentration gradient, driven by the random motion of particles.
Diffusion is essential for the transport of nutrients, oxygen, and other essential molecules into cells and the removal of waste products.
Similarities between Osmosis and Diffusion
Osmosis and diffusion, while distinct processes, share several common features. Both involve the movement of molecules across a semipermeable membrane, which acts as a barrier that selectively allows certain substances to pass through while blocking others.
Molecule Movement
In both osmosis and diffusion, molecules move from an area of higher concentration to an area of lower concentration. This movement occurs through passive transport, meaning that it does not require energy input from the cell.
Membrane Permeability
The semipermeable membrane plays a crucial role in both osmosis and diffusion. It allows water molecules and small, nonpolar molecules to pass through freely, while blocking the passage of larger molecules and ions. This selective permeability is essential for maintaining the proper functioning of cells.
Differences between Osmosis and Diffusion: Osmosis And Diffusion Venn Diagram
Osmosis and diffusion share the common theme of movement across a selectively permeable membrane, but they exhibit distinct differences. Osmosis, a specific form of diffusion, involves the movement of water molecules, while diffusion encompasses the movement of any type of molecule.
Another key distinction lies in the role of concentration gradients. In osmosis, water molecules move from an area of low solute concentration to an area of high solute concentration, driven by the concentration gradient of the dissolved particles. In contrast, diffusion involves the movement of molecules from an area of high concentration to an area of low concentration, regardless of the nature of the molecules.
Venn Diagram of Osmosis and Diffusion
Osmosis and diffusion are two fundamental processes in biology that involve the movement of molecules across a selectively permeable membrane. While both processes share some similarities, they also have distinct differences. A Venn diagram can help visualize these similarities and differences.
The Venn diagram below illustrates the key similarities and differences between osmosis and diffusion:
Similarities:
- Both osmosis and diffusion are passive processes, meaning they do not require energy input.
- Both processes involve the movement of molecules across a selectively permeable membrane.
- Both processes can occur in both living and non-living systems.
Differences:
- Osmosis is the movement of water molecules across a selectively permeable membrane, while diffusion is the movement of any molecule across a selectively permeable membrane.
- Osmosis is driven by a difference in water potential, while diffusion is driven by a difference in concentration.
- Osmosis can cause a change in cell volume, while diffusion does not.
| Characteristic | Osmosis | Diffusion |
|---|---|---|
| Type of molecules | Water molecules | Any molecule |
| Driving force | Water potential | Concentration |
| Effect on cell volume | Can change cell volume | Does not change cell volume |
Examples of Osmosis and Diffusion in Biological Systems
Osmosis and diffusion are fundamental processes that occur in all living organisms, playing vital roles in maintaining homeostasis and facilitating various biological functions. Here are some examples of osmosis and diffusion in biological systems:
Osmosis, Osmosis and diffusion venn diagram
- Water absorption by plant roots:Plant roots absorb water from the soil through osmosis. The root cells have a higher concentration of solutes than the surrounding soil, creating an osmotic gradient. Water molecules move from the soil into the root cells to equalize the solute concentration, leading to the absorption of water by the plant.
- Cell turgidity:Osmosis is responsible for maintaining cell turgidity, the state of fullness and rigidity of a cell. When a cell is placed in a hypotonic solution (a solution with a lower solute concentration than the cell), water moves into the cell by osmosis, causing it to swell and become turgid.
In contrast, when a cell is placed in a hypertonic solution (a solution with a higher solute concentration than the cell), water moves out of the cell, causing it to shrink and become flaccid.
Diffusion
- Gas exchange in lungs:Oxygen and carbon dioxide are exchanged between the lungs and the blood through diffusion. The alveoli in the lungs have a thin membrane that allows for the diffusion of gases. Oxygen from the inhaled air diffuses into the blood, while carbon dioxide from the blood diffuses into the exhaled air.
- Nutrient absorption in the digestive system:Nutrients are absorbed from the small intestine into the bloodstream through diffusion. The walls of the small intestine have numerous tiny finger-like projections called villi, which increase the surface area for absorption. Nutrients from the digested food diffuse across the villi and into the bloodstream.
Real-World Applications
Osmosis and diffusion have numerous practical applications in various fields, including medicine and biotechnology:
- Dialysis:Osmosis is used in dialysis, a medical procedure that removes waste products from the blood of patients with kidney failure. Dialysis involves using a semipermeable membrane to separate the waste products from the blood, allowing them to diffuse out of the body.
- Drug delivery:Diffusion is used in drug delivery systems to control the release of drugs into the body. Drug delivery systems are designed to release drugs over a specific period, ensuring optimal drug concentration in the body.
- Biotechnology:Osmosis and diffusion are used in various biotechnology applications, such as the production of biofuels and pharmaceuticals. In biofuel production, osmosis is used to extract sugars from plant biomass, which are then fermented to produce biofuels. In pharmaceutical production, diffusion is used to separate and purify drug molecules.
Factors Affecting Osmosis and Diffusion
The rate of osmosis and diffusion is influenced by several factors, including temperature, concentration gradients, and surface area. Understanding these factors is crucial for comprehending the dynamics of these processes in biological systems.
Temperature
Temperature plays a significant role in osmosis and diffusion. As temperature increases, the kinetic energy of molecules increases, leading to faster movement and higher rates of both processes. This is because higher temperatures provide more energy for molecules to overcome the energy barrier required for movement across a selectively permeable membrane or from an area of high concentration to an area of low concentration.
Concentration Gradients
Concentration gradients are another important factor affecting osmosis and diffusion. The greater the concentration gradient, the faster the rate of diffusion. This is because a larger concentration gradient provides a stronger driving force for molecules to move from an area of high concentration to an area of low concentration.
In the case of osmosis, a higher concentration gradient of solutes across a selectively permeable membrane results in a faster rate of water movement to equalize the solute concentrations on both sides of the membrane.
Surface Area
The surface area available for diffusion or osmosis also affects the rate of these processes. The larger the surface area, the faster the rate of diffusion or osmosis. This is because a larger surface area provides more space for molecules to move across, facilitating a more rapid exchange of substances.
FAQ Corner
What is the primary difference between osmosis and diffusion?
Osmosis involves the movement of water molecules across a semipermeable membrane, while diffusion encompasses the movement of any type of molecule across a concentration gradient.
How does temperature affect osmosis and diffusion?
Increased temperature accelerates both osmosis and diffusion rates, as it increases the kinetic energy of molecules.
Provide an example of osmosis in everyday life.
When you place a raisin in water, it swells due to the influx of water molecules via osmosis.