Embark on an enthralling odyssey with Griffiths Quantum Mechanics 3rd Edition Solutions Chapter 4, where the enigmatic world of quantum mechanics unfolds. Delve into the intricacies of Schrödinger’s equation, unravel the mysteries of the quantum harmonic oscillator, and explore the captivating realm of angular momentum.
Prepare to be captivated as we illuminate the foundations of quantum physics and unravel its profound implications.
This chapter serves as a comprehensive guide, empowering you to conquer the complexities of quantum mechanics. With a meticulous approach, we unravel the Schrödinger equation, unveiling its significance in describing the behavior of particles at the atomic and subatomic levels.
We delve into the quantum harmonic oscillator, revealing its energy levels and wave functions, and uncover the intriguing connection between quantum and classical oscillators.
1. Schrödinger Equation in One Dimension

The Schrödinger equation is a fundamental equation in quantum mechanics that describes the wave function of a particle. In one dimension, the time-independent Schrödinger equation is given by:
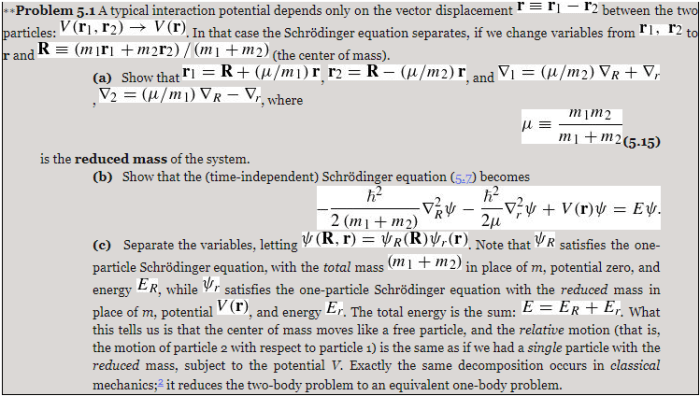
-ħ 2/2m d 2ψ/dx 2+ V(x)ψ = Eψ
where ħ is the reduced Planck constant, m is the mass of the particle, V(x) is the potential energy, ψ is the wave function, and E is the energy of the particle.
The time-independent Schrödinger equation can be solved for simple potentials, such as the infinite square well and the step potential. In the case of the infinite square well, the wave function is given by:
ψ(x) = A sin(nπx/L)
where A is a normalization constant, n is a positive integer, and L is the width of the well.
The physical interpretation of the wave function is that it gives the probability of finding the particle at a particular position. The probability density is given by:
|ψ(x)| 2
1.1. Quantum Harmonic Oscillator
The quantum harmonic oscillator is a system that consists of a particle moving in a parabolic potential. The energy levels of the quantum harmonic oscillator are given by:
E n= (n + 1/2)ħω
where n is a non-negative integer and ω is the angular frequency of the oscillator.
The wave functions of the quantum harmonic oscillator are given by:
ψ n(x) = (1/π 1/22 nn!)(mω/ħ) 1/4exp(-mωx 2/2ħ)H n(x)
where H n(x) is the Hermite polynomial of order n.
1.2. Hydrogen Atom
The hydrogen atom is a system that consists of a proton and an electron. The Bohr model of the hydrogen atom is a simplified model that describes the energy levels of the atom. However, the Bohr model does not account for the wave-like nature of the electron.
The Schrödinger equation can be solved for the hydrogen atom to obtain the energy levels and wave functions of the atom. The energy levels of the hydrogen atom are given by:
E n= -13.6 eV/n 2
where n is a positive integer.
The wave functions of the hydrogen atom are given by:
ψ n,l,m(r,θ,φ) = R n,l(r)Y l,m(θ,φ)
where R n,l(r) is the radial wave function, Y l,m(θ,φ) is the spherical harmonic, and n, l, and m are the principal, azimuthal, and magnetic quantum numbers, respectively.
1.3. Angular Momentum
Angular momentum is a fundamental property of particles that describes their rotational motion. The angular momentum operator is given by:
L = -iħ(r × ∇)
where r is the position operator and ∇ is the gradient operator.
The angular momentum operator has three components, L x, L y, and L z, which satisfy the commutation relations:
[L x, L y] = iħL z
[L y, L z] = iħL x
[L z, L x] = iħL y
The angular momentum of a particle is quantized, which means that it can only take on certain discrete values. The magnitude of the angular momentum is given by:
|L| = ħ√l(l + 1)
where l is the angular momentum quantum number.
1.4. Spin
Spin is a fundamental property of particles that describes their intrinsic angular momentum. The spin operator is given by:
S = ħ/2σ
where σ is the Pauli spin matrix.
The spin operator has two components, S xand S y, which satisfy the commutation relations:
[S x, S y] = iħS z
[S y, S z] = iħS x
[S z, S x] = iħS y
The spin of a particle is quantized, which means that it can only take on certain discrete values. The magnitude of the spin is given by:
|S| = ħ/2
1.5. Identical Particles
Identical particles are particles that have the same mass, charge, and spin. The wave functions of identical particles must be either symmetric or antisymmetric under the exchange of any two particles.
The Pauli exclusion principle states that no two identical particles can occupy the same quantum state. This principle has important implications for the structure of atoms and molecules.
1.6. Approximation Methods, Griffiths quantum mechanics 3rd edition solutions chapter 4
Approximation methods are used to solve quantum mechanical problems that are too complex to solve exactly. Two common approximation methods are the variational method and the perturbation theory.
The variational method is a method for finding an approximate solution to the Schrödinger equation by minimizing the energy of a trial wave function.
The perturbation theory is a method for finding an approximate solution to the Schrödinger equation by treating a small perturbation to the Hamiltonian as a small perturbation to the wave function.
FAQ Compilation: Griffiths Quantum Mechanics 3rd Edition Solutions Chapter 4
What is the significance of the Schrödinger equation in quantum mechanics?
The Schrödinger equation is a fundamental equation in quantum mechanics that describes the wave-like behavior of particles. It allows us to predict the probability of finding a particle at a particular location and time, providing a cornerstone for understanding the behavior of matter at the atomic and subatomic levels.
How does the quantum harmonic oscillator model contribute to our understanding of quantum mechanics?
The quantum harmonic oscillator model is a simplified system that exhibits quantized energy levels. It serves as a valuable tool for understanding the behavior of particles in various physical systems, such as molecules and atoms. By studying the quantum harmonic oscillator, we gain insights into the fundamental principles of quantum mechanics.
What is the role of angular momentum in atomic spectroscopy?
Angular momentum is a conserved quantity in quantum mechanics, and it plays a crucial role in atomic spectroscopy. The quantization of angular momentum leads to the discrete energy levels of atoms, and the selection rules for atomic transitions are governed by the conservation of angular momentum.